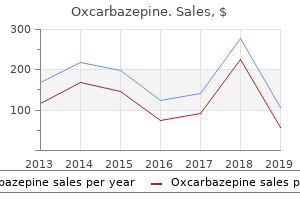
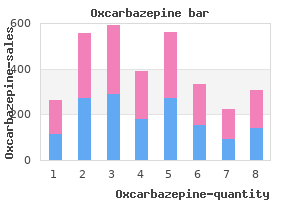
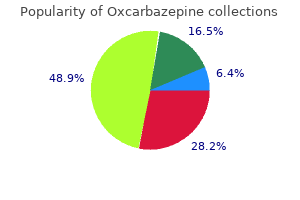
"150 mg oxcarbazepine sale, medications known to cause nightmares".
By: Z. Trompok, M.A., Ph.D.
Professor, Lincoln Memorial University DeBusk College of Osteopathic Medicine
In addition symptoms 4 dpo buy generic oxcarbazepine 150 mg on line, many of the factors that cause medications 44 175 cheap oxcarbazepine 150 mg without a prescription, or lead to medications at 8 weeks pregnant cheap 150 mg oxcarbazepine amex, a delay in the commencement or completion of clinical trials ultimately may lead to the denial of regulatory approval of a product candidate medications during breastfeeding cheap oxcarbazepine 600 mg amex. Clinical trials may produce negative or inconclusive results, and we or our partners may decide, or regulators may require us, to conduct additional clinical or preclinical testing. In addition, data obtained from tests are susceptible to varying interpretations, and regulators may not interpret our data as favorably as we do, which may delay, limit or prevent regulatory approval. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidate. Frequently, product candidates that have 37 Table of Contents shown promising results in early clinical trials have subsequently suffered significant setbacks in later clinical trials. In addition, the design of a clinical trial can determine whether its results will support approval of a product and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. We have limited experience in designing clinical trials and may be unable to design and execute a clinical trial to support regulatory approval. Further, clinical trials of potential products often reveal that it is not practical or feasible to continue development efforts. A number of companies in the pharmaceutical industry, including those with greater resources and experience than us, have suffered significant setbacks in Phase 3 clinical trials, even after seeing promising results in earlier clinical trials. In some instances, there can be significant variability in safety and/or efficacy results between different trials of the same product candidate due to numerous factors, including changes in trial protocols, differences in size and type of the patient populations, adherence to the dosing regimen, particularly for self-administered topical drugs, and the rate of dropout among clinical trial participants. We do not know whether any Phase 2, Phase 3 or other clinical trials we or any partners may conduct will demonstrate consistent and/or adequate efficacy and safety to obtain regulatory approval to market our product candidates. We have limited experience in preparing, submitting and prosecuting regulatory filings. We may require more time and incur greater costs than our competitors and may not succeed in obtaining regulatory approvals of products that we develop. Our product candidates may have undesirable side effects that may delay or prevent marketing approval, or, if approval is received, require them to be taken off the market or otherwise limit their sales. Unforeseen side effects from any of our product candidates could arise either during clinical development or, if approved, after the approved product has been marketed. For example, a small number of patients who received tavaborole treatment experienced some skin irritation around their toenails during clinical trials of tavaborole for onychomycosis. The range and potential severity of possible side effects from systemic therapies is greater than for topically administered drugs. Any delay in the regulatory review or approval of any of our product candidates will harm our business. All of our product candidates require regulatory review and approval prior to commercialization. Any delays in the regulatory review or approval of our product candidates would delay market launch, increase our cash requirements and result in additional operating losses. We cannot be certain that we or our partners will be able to respond to any regulatory requests during the review period in a timely manner without delaying potential regulatory action. In addition, delays in approvals or rejections of marketing applications may be based upon many factors, including regulatory requests for additional analyses, reports, data and studies, regulatory questions regarding data and results, changes in regulatory policy during the period of product development and the emergence of new information regarding our product candidates or other products. Furthermore, regulatory attitudes towards the data and results required to demonstrate safety and efficacy can change over time and can be affected by many factors, such as the emergence of new information, including on other products, policy changes and agency funding, staffing and leadership. We do not know whether future changes to the regulatory environment will be favorable or unfavorable to our business prospects. In addition, the environment in which our regulatory submissions may be reviewed changes over time. Review times can be affected by a variety of factors, including budget and funding levels and statutory, regulatory and policy changes. Moreover, in light of widely publicized events concerning the safety risk of certain drug products, regulatory authorities, members of Congress, the Government Accounting Office, medical professionals and the general public have raised concerns about potential drug safety issues. Our use of boron chemistry to develop pharmaceutical product candidates is novel and may not prove successful in producing approved products. Undesirable side effects of any of our product candidates, or of boron-based drugs developed by others, may extend the time period required to obtain regulatory approval or harm market acceptance of our product candidates, if approved. All of our product development activities are centered on compounds containing boron. If boron-based compounds developed by us or others have significant adverse side effects, regulatory authorities could require additional studies of our boron-based compounds, which could delay the timing of and increase the cost for regulatory approvals of our product candidates.
Other persons at risk include those receiving immunosuppressive therapy (particularly glucocorticoids) for cancer and organ transplantation; those taking biologic agents treatment for sciatica order oxcarbazepine 150 mg on-line. The organisms are inhaled and attach tightly to type I cells in alveoli schedule 9 medications buy 150 mg oxcarbazepine with mastercard, although they remain extracellular symptoms wheat allergy purchase oxcarbazepine 600mg on-line. On physical examination medicine during the civil war purchase oxcarbazepine 300 mg, pts are found to have tachypnea, tachycardia, and cyanosis, but findings on pulmonary examination are often unremarkable. Serum lactate dehydrogenase levels can be elevated, but this finding is nonspecific. Chest x-ray classically reveals bilateral diffuse infiltrates beginning in the perihilar regions. Methenamine silver and other cell wall stains selectively stain the wall of Pneumocystis cysts. Over time, pts are rendered immune to disease but remain susceptible to infection. Cerebral malaria: coma, obtundation, delirium, diffuse symmetric encephalopathy without focal neurologic signs. Poor prognostic signs: hypoglycemia, especially in children and pregnant women (may be exacerbated by quinine or quinidine treatment); acidosis; noncardiogenic pulmonary edema; renal failure; severe anemia and coagulation abnormalities; severe jaundice and liver dysfunction Malaria in pregnancy: Pregnant women have unusually severe illness. Transfusion malaria: has a shorter incubation period than naturally acquired disease Tropical splenomegaly: abnormal response to repeated infections. Thick smears concentrate parasites by 40- to 100-fold compared with thin smears and increase diagnostic sensitivity. These agents are not available in the United States for treatment of uncomplicated P. Exchange transfusions can be considered for severely ill pts, although indications for their use are not yet agreed upon. Clindamycin (10 mg/kg bid for 7 days) or Atovaquone-proguanil (20/8 mg/kg qd for 3 days with food) (continued) Regimen(s) Sensitive P. The data from large studies in Southeast Asia showed a 35% reduction in mortality rate from that with quinine. Severe malaria in children in high-transmission settings has different characteristics; thus trials are ongoing in Africa comparing artesunate with quinine to determine whether there is a survival benefit in African children. Take daily at the same time each day while in the malarious area and for 7 days after leaving such areas. Atovaquone-proguanil is contraindicated in persons with severe renal impairment (creatinine clearance rate <30 mL/min). It is not recommended for children weighing <5 kg, pregnant women, or women breastfeeding infants weighing <5 kg. Take daily at the same time each day while in the malarious areas and for 4 weeks after leaving such areas. Take weekly on the same day of the week while in the malarious areas and for 4 weeks after leaving such areas. Mefloquine is contraindicated in persons allergic to this drug or related compounds. Use with caution in persons with psychiatric disturbances or a history of depression. Take daily at the same time each day while in the malarious areas and for 7 days after leaving such areas. Epidemiology In the United States, infections occur most frequently along the northeastern coast. There is a gradual onset of fevers, fatigue, myalgias, arthralgias, and-less often-dyspnea, headache, anorexia, and nausea. Pts have mild hepatosplenomegaly, anemia, thrombocytopenia, and elevated liver enzymes. Parasitemia levels may range from 1 to 10% in immunocompetent pts and up to 85% in asplenic pts.
Oxcarbazepine 300mg online. Symptoms of a Sinus Infection Explained | Sinus Doctor.
More recently treatment for hemorrhoids order 150mg oxcarbazepine fast delivery, nonmyeloablative conditioning regimens have been used with better outcomes symptoms 24 hours before death cheap oxcarbazepine 150mg otc. To date severe withdrawal symptoms generic oxcarbazepine 300mg line, the published experience remains primarily in case reports or small series treatment anemia effective oxcarbazepine 150mg. These regimens are associated with lower toxicity, rapid engraftment, and potentially lower posttransplantation infectious complications. Most of these reports detail incomplete donor chimerism but relatively good outcome with resolution of enteritis, diabetes, and other pretransplantation complications. The precise degree of chimerism required for successful engraftment is unknown, but considering that the host immune system appears to have normal effector function, sustained engraftment of only the Treg cell compartment has been speculated to be sufficient for successful long-term reconstitution. Complement deficiency should be considered in the evaluation of patients with autoimmune disease. We mention here only that many complement component deficiencies are associated with a significant propensity toward autoimmune disease. Complement function should be considered in patients presenting with autoimmune disease. Phagocytic cell defects the general approach to the diagnosis and evaluation of suspected phagocytic cell disorders is summarized in Fig E4. Periodontitis can accompany oral ulceration and gingivitis; vaginal and rectal mucosal ulcers are also seen. The severity of the infectious complications tends to parallel the severity of the neutropenia. Additional genetic lesions have recently been identified in patients with various syndromes in which neutropenia is a component. Inflammatory bowel disease is frequently seen and is thought to be secondary to defective leukocytes. The periodicity of cyclic neutropenia is usually about 21 days but can range from 14 to 36 days. Infections occur only during the nadirs of the neutrophil count, but there is a lag between the nadir of the neutrophil count and the onset of clinical symptoms so that quite often neutrophil counts are normal when the patients are seen for symptoms. Patients with severe chronic neutropenia (but not those with cyclic neutropenia) have an increased incidence of acute myeloid leukemia or myeloid dysplasia. Long-term follow-up data from the Severe Chronic Neutropenia International Registry found an incidence of acute myeloid leukemia/myeloid dysplasia of 2. Other pyogenic infections are not as severe as in the classical form, and patients might not receive a diagnosis until childhood or later. When bacterial infection is present, neutrophil counts can increase to as high as 100,000 cells/mm3. These patients are sometimes thought to have myeloid leukemia or leukemoid reactions. Discontinuation of fucose supplements results in a rapid loss of selectin ligands and increases in peripheral neutrophil counts. Skin infections are usually indolent, and severe infections with abscess formation can also affect the lungs, lymph nodes, ears, and mastoids. The specific granules are devoid of most of their contents and are not visible after Wright staining. Additional genetic lesions should be investigated in patients with clinical and laboratory features consistent with neutrophil defects who are not found to have any of the disorders listed previously. Granulomatous abscesses occur in the lungs (approximately 75% of patients), lymph nodes (50%), skin (40%), liver (25%), and bones (25%). The principal bacterial pathogens are usually catalase producing and include S aureus and Salmonella, Klebsiella, Aerobacter, Serratia, Nocardia, and Burkholderia species. Infection with Aspergillus fumigatus occurs in a majority of patients; C albicans is another prominent fungal pathogen. Granulomatous inflammation can lead to obstruction of the stomach, ureter, or esophagus in some patients. Physical examination can reveal growth failure, evidence of abscesses or other infection in any region, or lymphadenopathy, organomegaly, or both. Although most patients are affected as infants or young children, adults occasionally present with acute severe fungal pneumonia.
Piperacillin/tazobactam or ticarcillin/clavulanate has little more activity against P symptoms ulcerative colitis generic oxcarbazepine 600mg line. The antipseudomonal activity of cefepime is equivalent to that of ceftazidime treatment 4 lung cancer order oxcarbazepine 150 mg on-line, with less potential for -lactamase induction in gram-negative enteric bacteria symptoms parkinsons disease oxcarbazepine 300mg otc. Colistin inhalational therapy consists of 75 mg in 3 mL of normal saline via nebulizer medications for ptsd generic 150mg oxcarbazepine otc, given twice daily. Risk factors include prolonged hospitalization, malignancy and neutropenia, instrumentation, and prior therapy with broad-spectrum antibiotics. Glanders is associated with close contact with horses or other equines and is caused by Burkholderia mallei. These diseases present as acute or chronic pulmonary or extrapulmonary suppurative illnesses or as acute septicemia. Epidemiology Legionella is found in fresh water and human-constructed water sources. Outbreaks have been traced to cooling towers and potable-water distribution systems. Pts who have chronic lung disease, smoke, and/or are elderly or immunosuppressed are at particular risk for disease. Clinical Features monia does not develop, but malaise, fatigue, myalgias, and fever are frequent. The heart is the most common extrapulmonary site of disease (myocarditis, pericarditis, and occasionally prosthetic valve endocarditis). Urinary antigen is detectable 3 days after disease onset, with positive results persisting for weeks. Prognosis Mortality approaches 80% among compromised hosts who do not receive timely therapy. Brucellosis is transmitted via exposure to infected animals or their products in either occupational settings. Brucellosis can present as a febrile illness similar to but less severe than typhoid fever. However, the most common findings are musculoskeletal pain and involvement of the peripheral and axial skeleton- i. Brucella infection can cause lymphadenopathy, hepatosplenomegaly, and focal abscess. Brucellosis must be distinguished from tuberculosis; if this distinction is not possible, the regimen should be tailored to avoid inadvertent monotherapy for tuberculosis (see below). Brucellosis tends to cause less bone and joint destruction than tuberculosis (Table 99-1). Lymphadenopathy is related to the location of the tick bite; inguinal/ femoral nodes are most often affected in adults because of the frequency of bites on the legs. Fleabites or direct contact with infected tissues or airborne droplets can cause human infections. Edema and erythema- but not cellulitis or lymphangitis- develop in the surrounding tissue.
© 2020 Vista Ridge Academy | Powered by Blue Note Web Design